Did I Have It?
This is the question many of us are asking. As our society adapts to living in a world with Sars-CoV-2, the virus responsible for the COVID-19 illness, efforts have turned to not only testing to see if someone is currently infected, but also testing that can help us determine if someone has had the infection in the past. This has the potential to open the doors for safely reintegrating people into normal society without the risk of transmitting the virus. Here is what you need to know about this testing, including some potential problems.
What is the difference between the antibody test and the diagnostic test used for COVID-19?
The diagnostic tests that are used primarily at hospitals and drive-through testing sites are tests to determine if you have an active infection. It is typically performed by nasopharyngeal swab where a long Q-tip is inserted deep into the nasal passages and a test looks for pieces of the virus itself called antigens with a process called PCR. This test will only tell you if you are currently infected and not if you had it in the past.
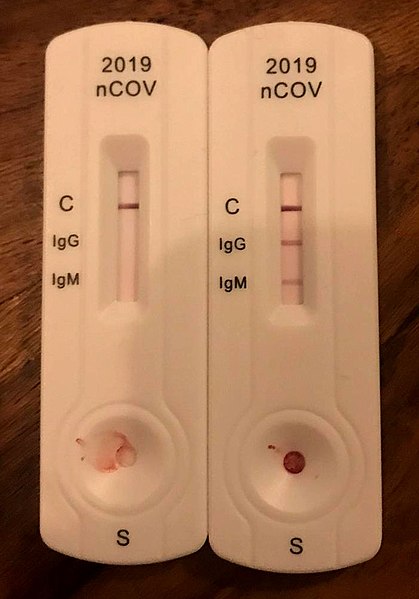
Image credit: Creative Commons Attribution-Share Alike 4.0 International , Author: Partynia
Antibody testing can help determine if you had the virus in the past and therefore may have immunity to the virus. Rather than looking for a piece of the virus, it is looking to see if you have developed a specific immune response to the virus called antibodies. Several ways of performing this test have emerged but many companies are favoring a finger stick test that can be performed at home.
What is an antibody?
Antibodies are immune proteins also called immunoglobulins (Ig) that recognize a specific structure found on the outside of foreign invaders to help promote an immune response. These specific structures are antigens, and in this case the foreign invader is a virus.
The antibody response is part of your adaptive immune system, meaning after seeing this foreign invader it adapts by learning and remembering this pathogen so that when it sees it again, your immune system can mount a rapid response specific to the invader. In many cases, this can mean you are immune to it and will not be infected again, however we don’t know if this is true for COVID-19.
What are IgG and IgM antibodies?
There are different types of antibodies (immunoglobulins) in your body including Immunoglobulin G (IgG) and Immunoglobulin M (IgM) which are particularly helpful in determining if you have had an infection. Some testing companies are also offering IgA and other markers but IgG and IgM by far get the most attention. IgM is a quicker response against foreign invaders, while the more prevalent IgG antibody provides longer term immunity.
There is still a lot we don’t know about Sars-CoV-2 antibodies, but according to one recent study, IgG and IgM antibodies were found within 19 days after a Sars-CoV-2 exposure in 100% of samples, with IgM appearing either first, or simultaneously with IgG, and both plateauing after 6 days. (1) IgM levels then begin to decline as IgG persists as a longer term marker of immune response.
Is this test available now?
Many companies are coming out with Sars-CoV-2 antibody tests, some of which are available now, but that doesn’t mean you should rush to get them…
What are some concerns with the antibody test?
- Problems with accuracy. Sensitivity and specificity are two primary means of measuring accuracy of a test. Sensitivity is how well the test detect positive results or people who have had the infection. Specificity is the test’s ability to identify negative results or those who have not had the infection.We don’t know if these tests have adequate sensitivity and specificity for the Sars-CoV-2 virus. If the test isn’t accurate, it could cause more harm than good. If you are told you have the antibodies, you may be more likely to resume normal activities, but what if the test has a 10% false positive rate? That means a 10% chance you actually don’t have the antibodies and could still be at risk. What if it cross-reacted with a different coronavirus and you have immunity to that virus, but not Sars-CoV-2? We need tests that are accurate and reliable before they are done on a mass scale.
- Immunity Passports have a predictive value problem. An immunity passport is the name given to the form of identification some countries are using to allow work and travel to people who have “proof” via antibody testing that they already had COVID-19. The major problem? A LOT of people are likely going to be given an immunity passport who have actually not had COVID-19. This is simply because that’s how math works, even when you have an accurate test. To read more about the importance of the predictive value of the test — the chance of actually having had COVID-19 if you have tested positive, read this article: https://blogs.scientificamerican.com/observations/beware-of-antibody-based-covid-19-immunity-passports/
- Many tests are lacking FDA approval – This means buyer beware! Companies can make and sell tests that are not up to standard. And once again, if the test isn’t accurate, it could cause more harm than good.
- Having the antibodies may not mean you are immune. With most viral infections, once you have the illness you are protected from getting it again. However, we do not yet know if this is true for COVID-19.
- We do not know how long IgG antibodies persist with COVID-19. At this point, all COVID-19 infections are relatively recent. Therefore, we don’t yet know how long IgG antibodies are likely to persist. Knowing how they persist is critical for correct interpretation of test results.
- You may still be contagious. Estimates of how long people continue to shed viral particles of Sars-CoV-2 range up to more than 6 weeks after symptom onset.(2) Your antibody test could tell you that you’ve had an infection but it doesn’t tell you whether you are still infectious or not.
Not all healthcare providers that are performing antibody tests are asking labs about their testing methodology, their sensitivity/specificity, and how the lab validated their results with confirmed cases. Results should also be reported by the lab to a health authority and should ideally be approved by the FDA.
Due to these issues, until more information is available, antibody tests should not be used to determine whether someone can safely return to normal activities without protective measures such as social distancing, shelter-in-place, mask usage, etc.
According to a statement by the American Association of Naturopathic Physicians,
“Companies offering serological testing are required by the FDA to inform providers and patients about the following:
- The test has not been reviewed by the FDA.
- Negative results do not rule out SARS-CoV-2 infection, particularly in those who have been in contact with the virus. Follow-up testing with a molecular diagnostic test should be considered to rule out infection in those individuals.
- Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection status.
- Positive results may be due to past or present infection with non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1, NL63, OC43, or 229E.
- More information about the current utility of serological testing can be found here as labs submit data to the FDA.”
The full statement can be found here.
Works Cited
- Long, Q., Liu, B., Deng, H. et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med (2020). https://doi.org/10.1038/s41591-020-0897-1
- Ai Tang Xiao, M.D, Yi Xin Tong, M.D, Ph.D, Sheng Zhang, M.D, Profile of RT-PCR for SARS-CoV-2: a preliminary study from 56 COVID-19 patients, Clinical Infectious Diseases, , ciaa460, https://doi.org/10.1093/cid/ciaa460

Leave A Comment